A COGNITIVE PARTNER FOR CLINICAL TRIALS
Vivo thinks alongside clinical teams to unify data, interpret signals, and surface what matters, in real time.
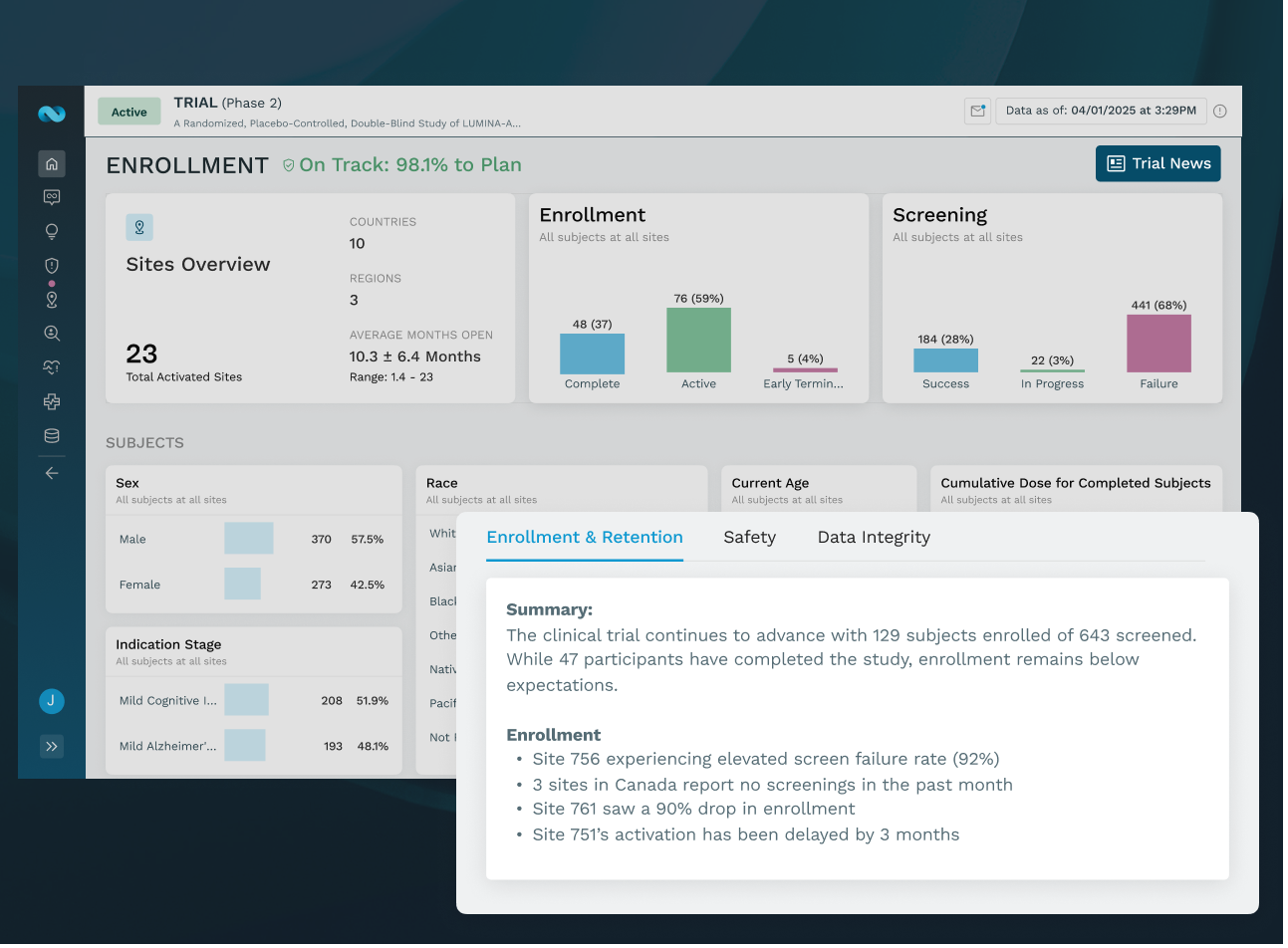
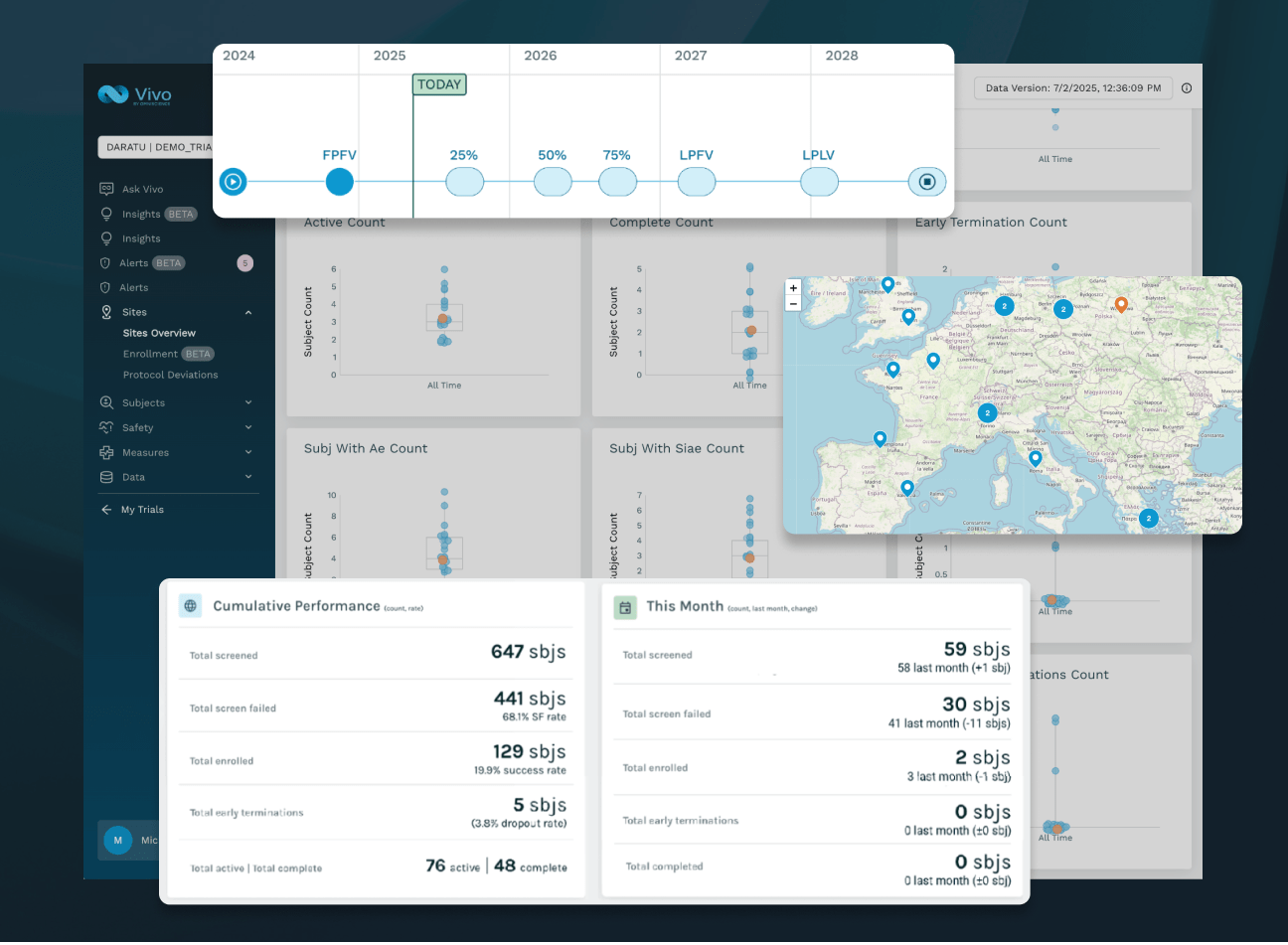
PORTFOLIO INTELLIGENCE, NOT JUST DASHBOARDS
See every trial, site, and signal in one intelligent view, identifying risk early and prioritizing action across trials.
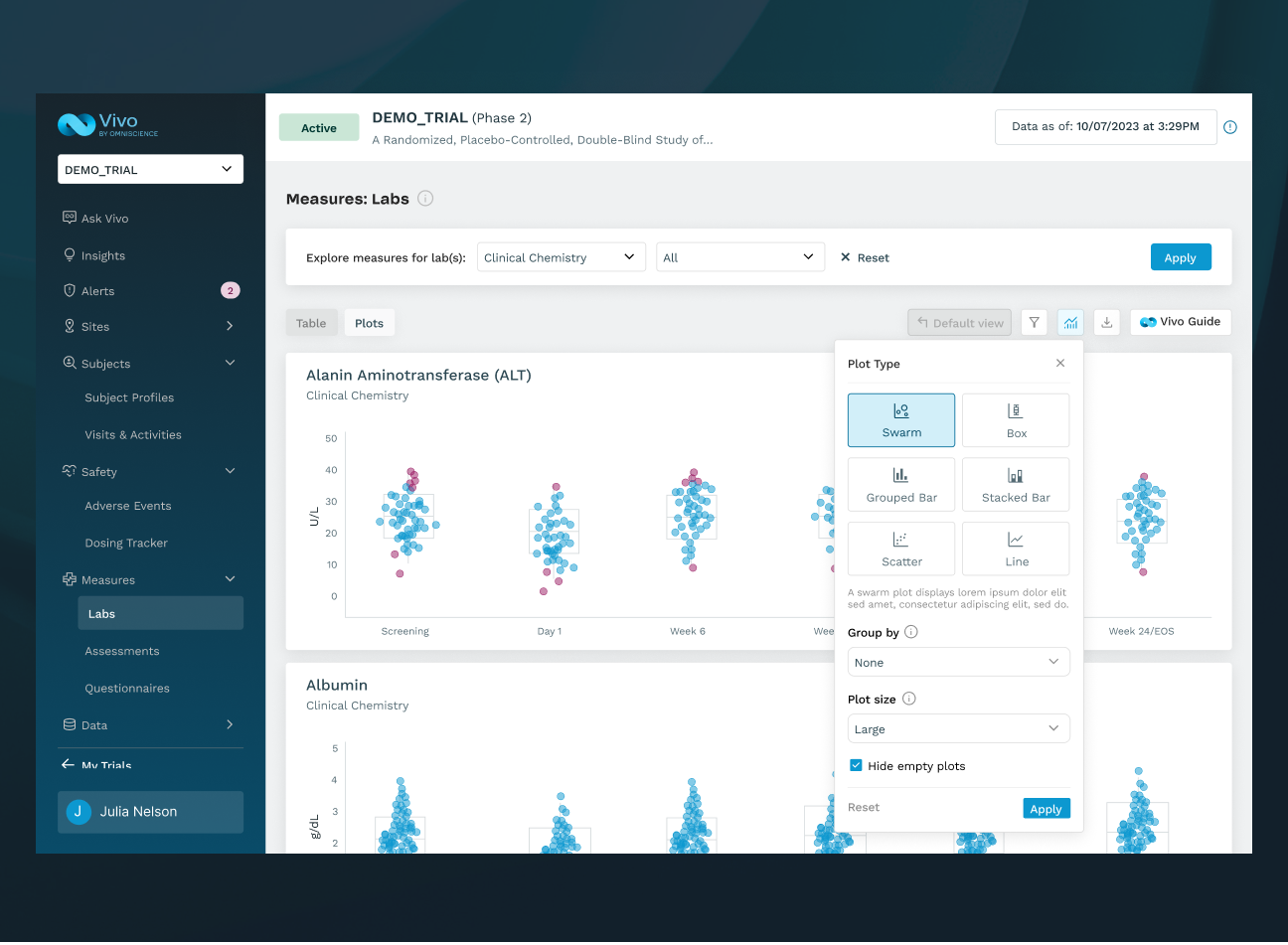
BUILT TO IMPROVE OUTCOMES AT SCALE
Vivo reduces manual effort, accelerates decisions, and helps teams deliver therapies to patients faster.